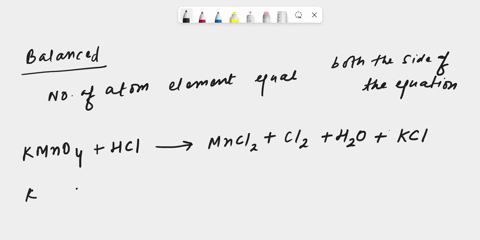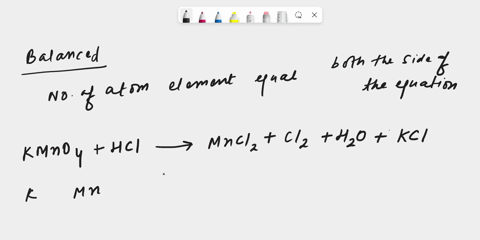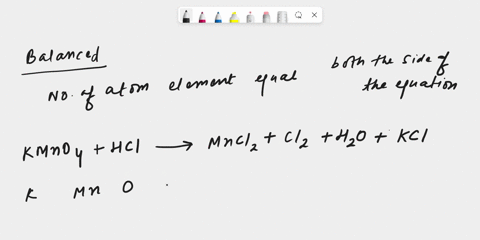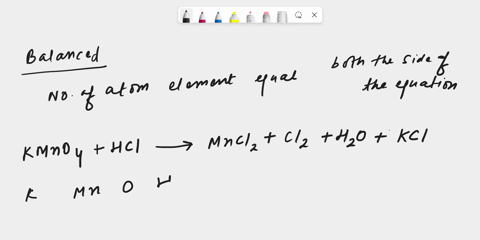
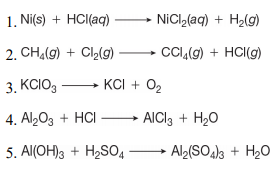
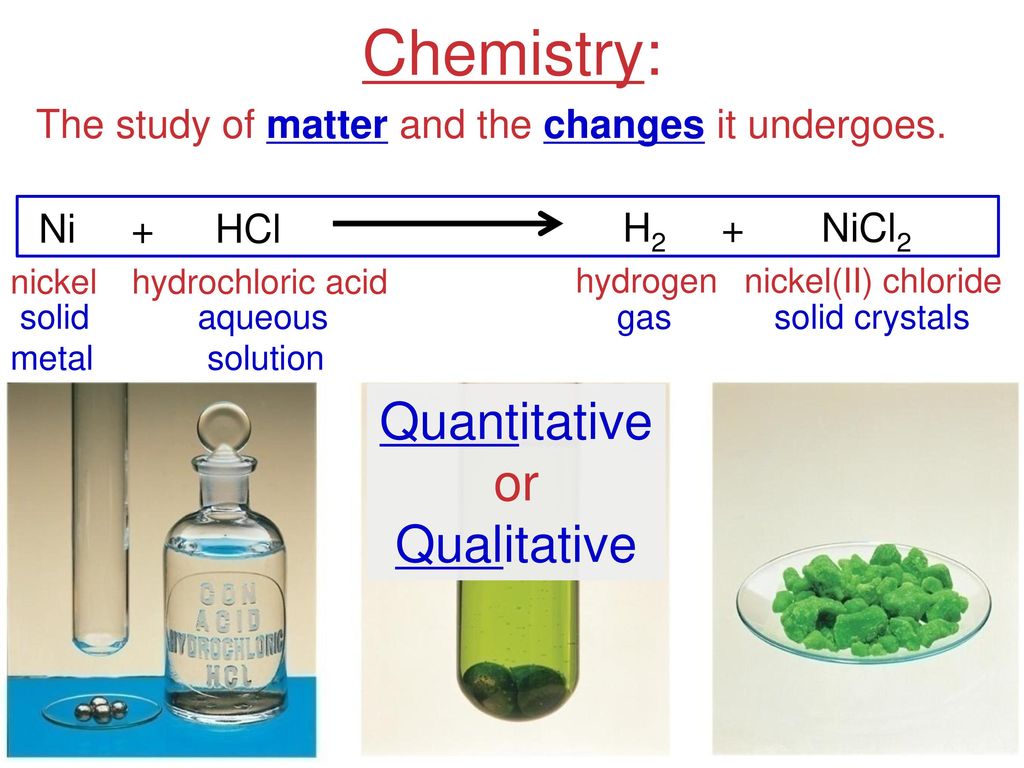
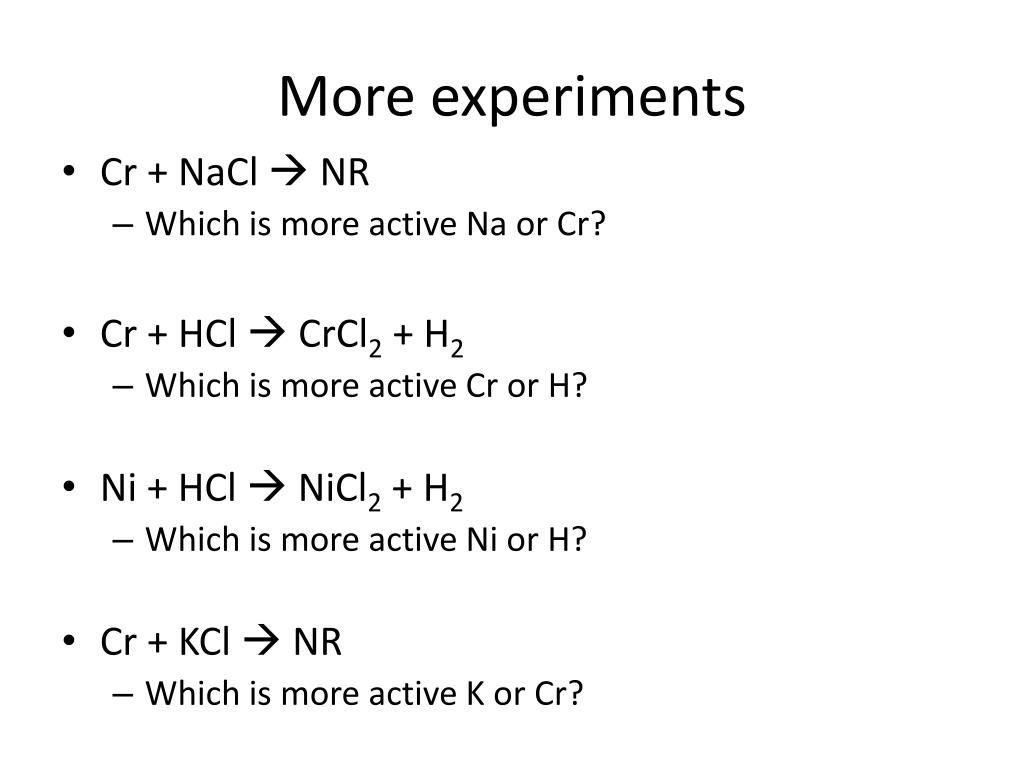
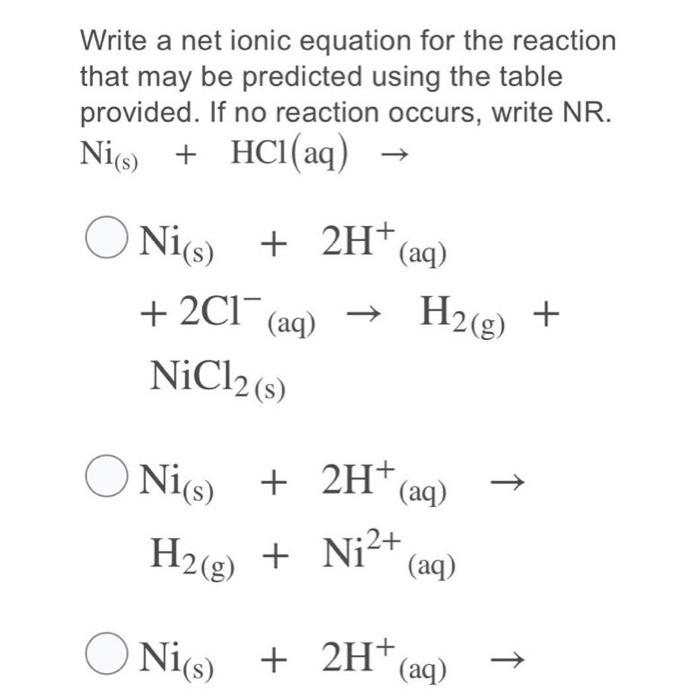
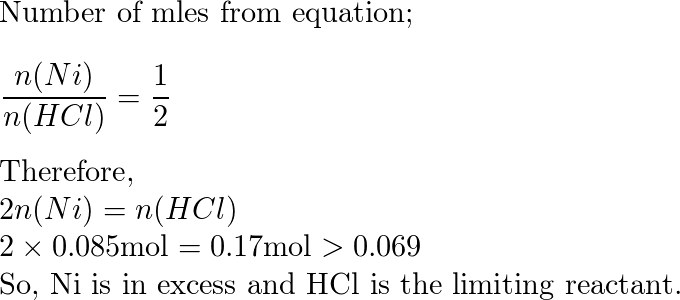inorganic chemistry - Explanation for why nickel turns green in hydrochloric acid - Chemistry Stack Exchange

Ethylene Dimerization and Oligomerization Using Bis(phosphino)boryl Supported Ni Complexes | Journal of the American Chemical Society
![On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into](https://i.ytimg.com/vi/Dx60N63HjFU/maxresdefault.jpg)
On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into