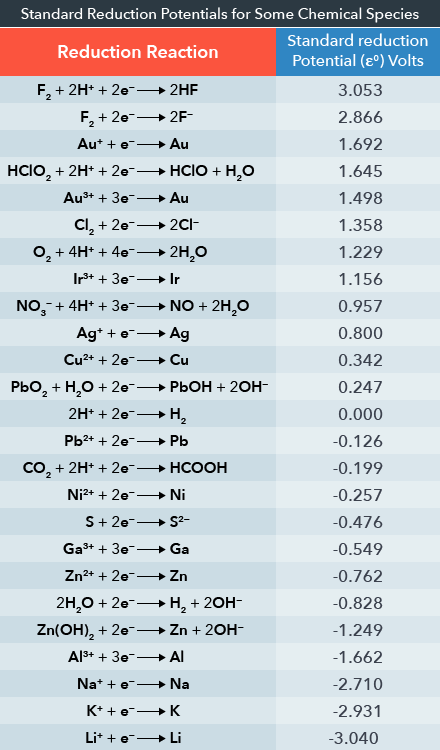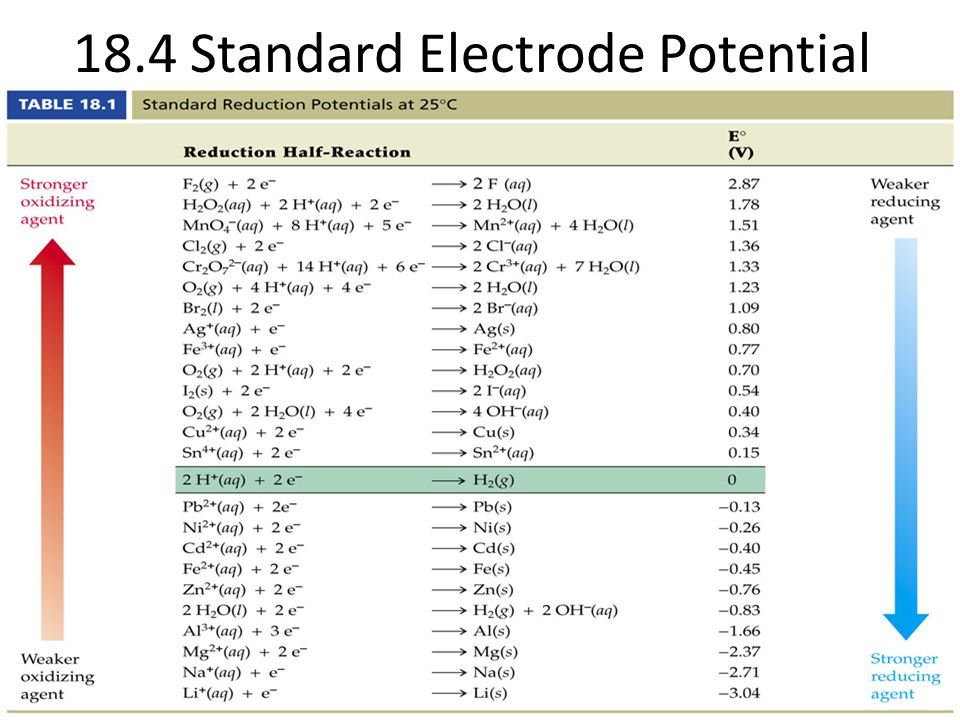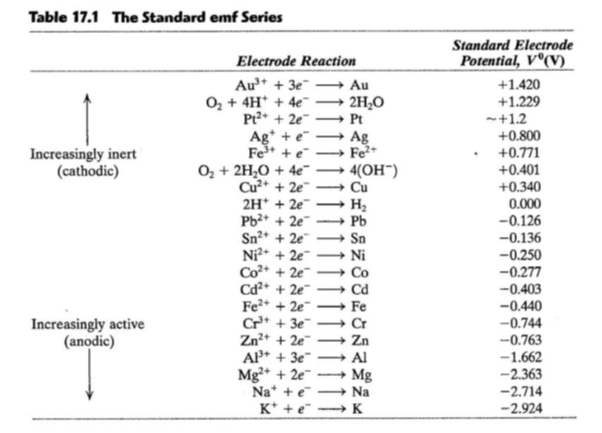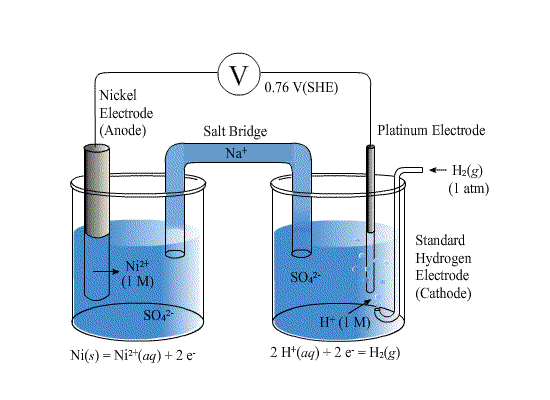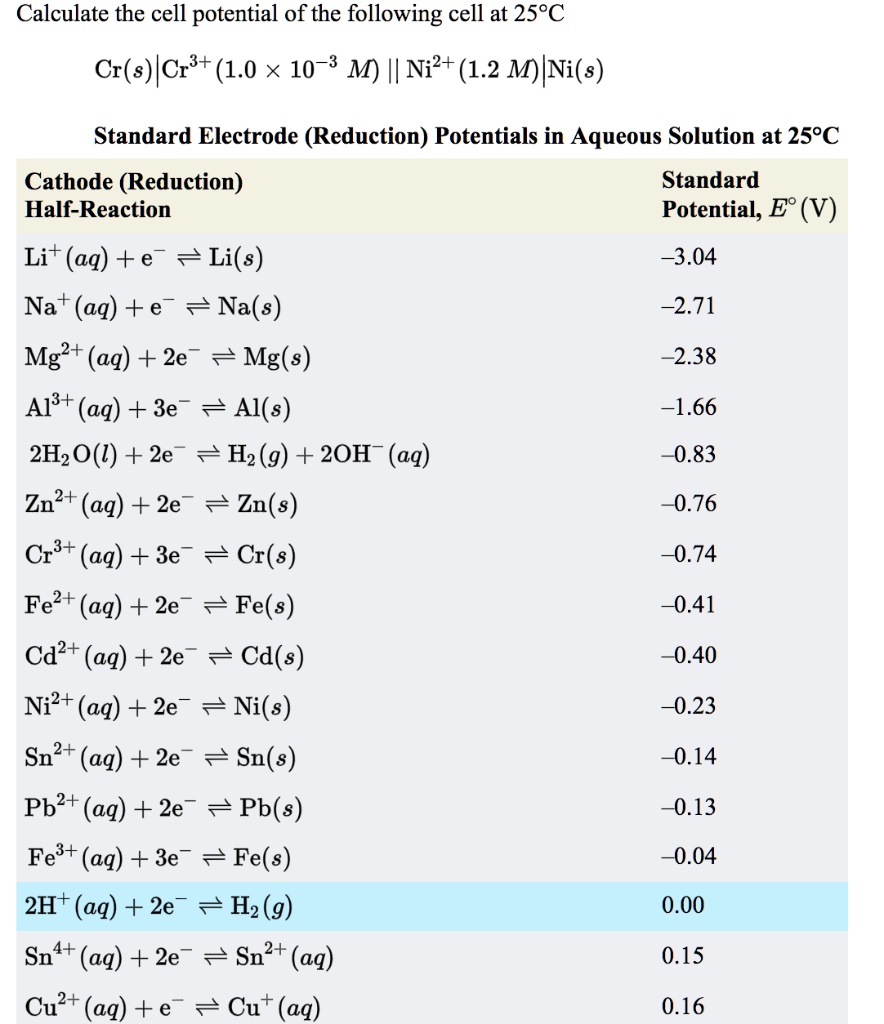
SOLVED: Calculate the cell potential of the following cell at 258C Cr(s)ICr8+(1.0 10-: M) || Ni2+ (1.2 M) Ni(s) Standard Electrode (Reduction) Potentials in Aqueous Solution at 259€ Cathode (Reduction) Standard Half-Reaction

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

The standard electrode potential of two half cells are given below - Ni^+2 + 2e^ - ⟶ Ni; E^0 = - 0.25V Zn^+2 + 2e^ - ⟶ Zn; E^0 = - 0.77V
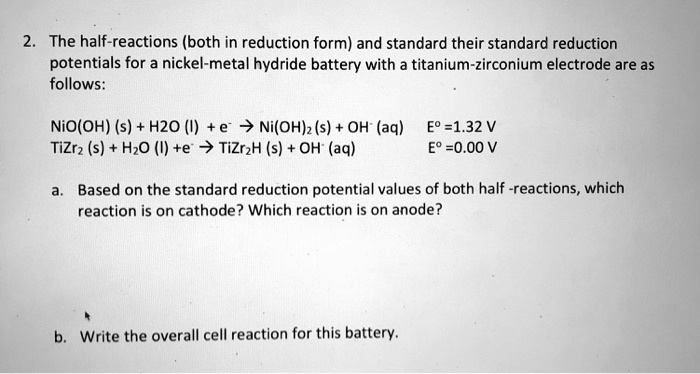
SOLVED: The half-reactions (both in reduction form) and standard their standard reduction potentials for nickel-metal hydride battery with titanium-zirconium electrode are as follows: NiO(OH) (s) - Hzo () 7 Ni(OHJz (s) +
The standard oxidation potential of Ni|Ni2+ electrode = 0.236 V. If this is combined with a hydrogen electrode in acid solution, at what pH of the solution will the measured emf be

Calculate the standard electrode potential of Ni2+/Ni electrode if |Class 12 CHEMISTRY | Doubtnut - YouTube

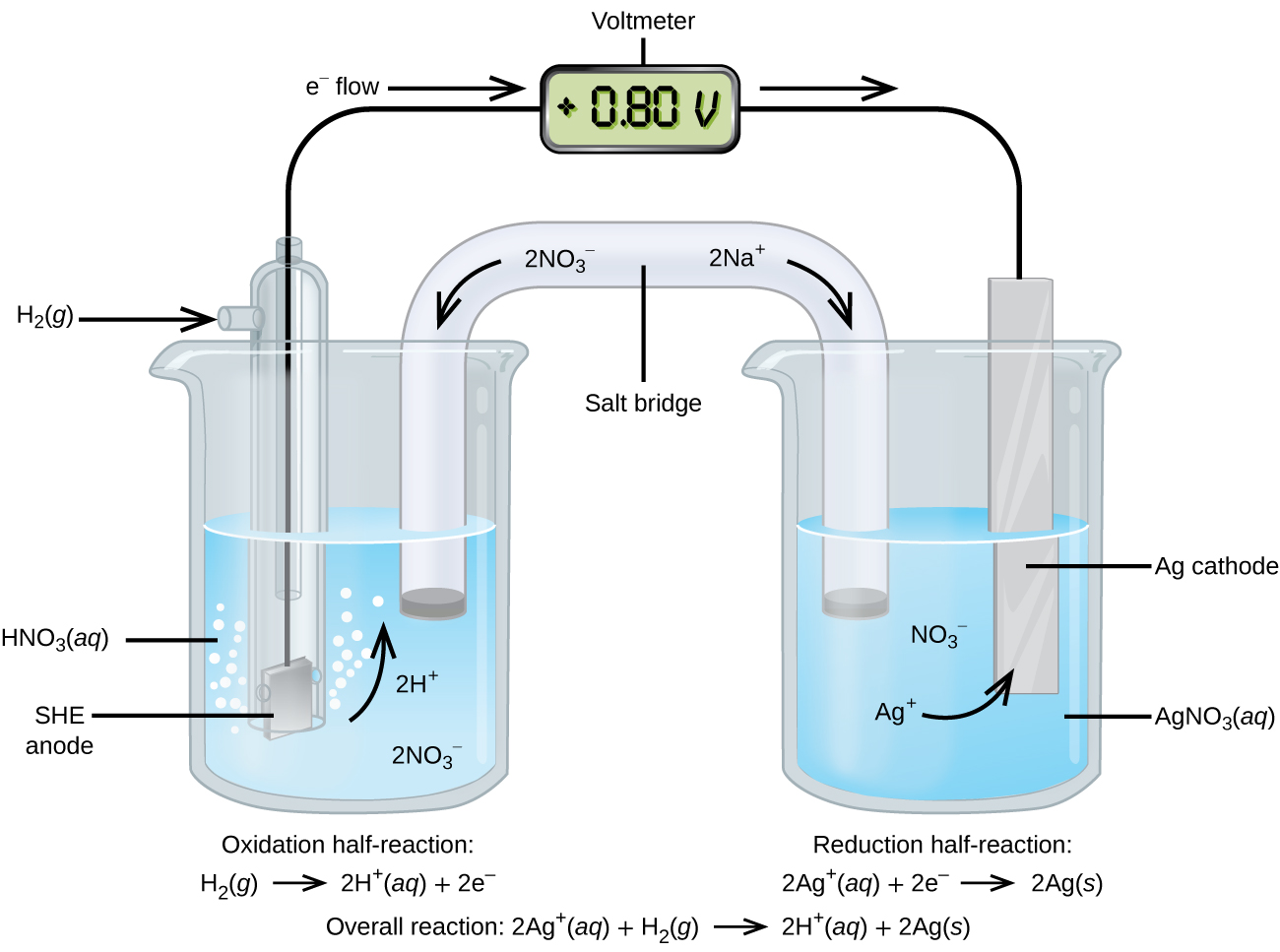
The EMF of the cell Ni `|Ni|Ni^(2+) ||Cu^(2+) |Cu(s)` is `0.59 ` volt. The standard reduction - YouTube
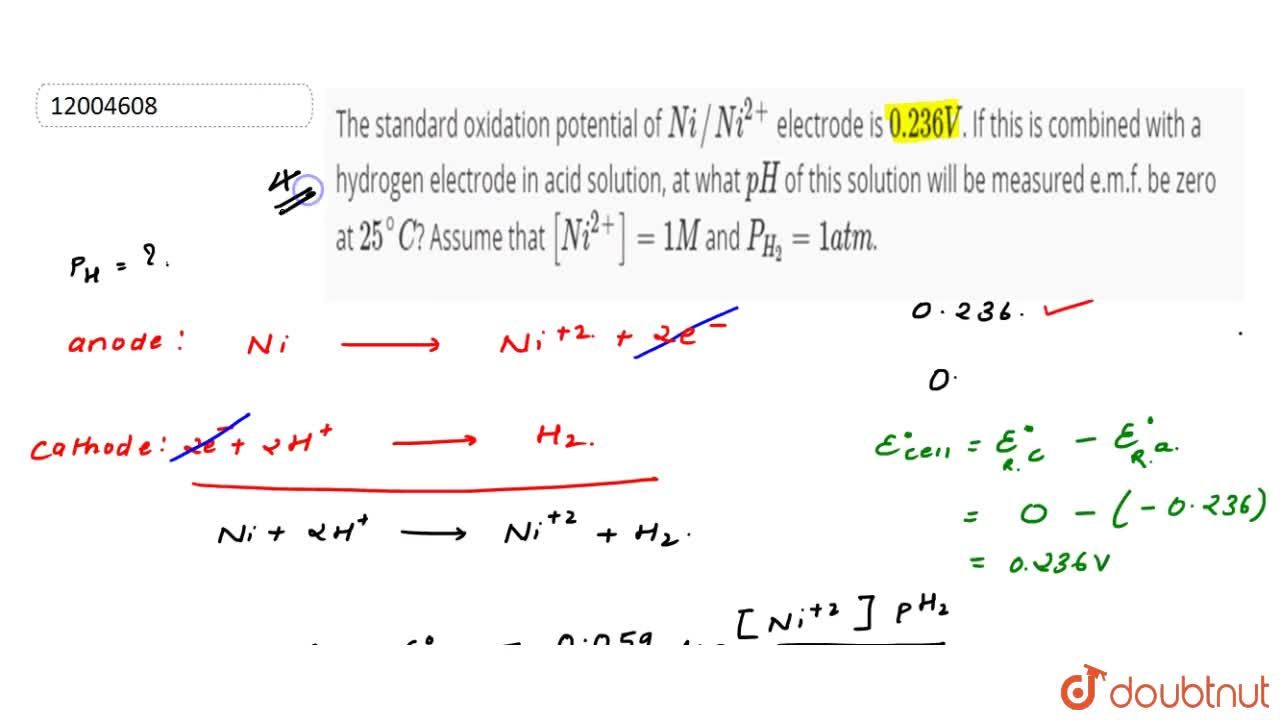
The standard oxidation potential of Ni//Ni^(2+) electrode is 0.236 V. If this is combined with a hydrogen electrode in acid solution, at what pH of this solution will be measured e.m.f. be

Ni | Ni^2 + || Cu^2 + | Cu The standard EMF of the above cell is 0.59 V. The standard electrode potential (reduction potential) of the copper electrode is 0.34 V.

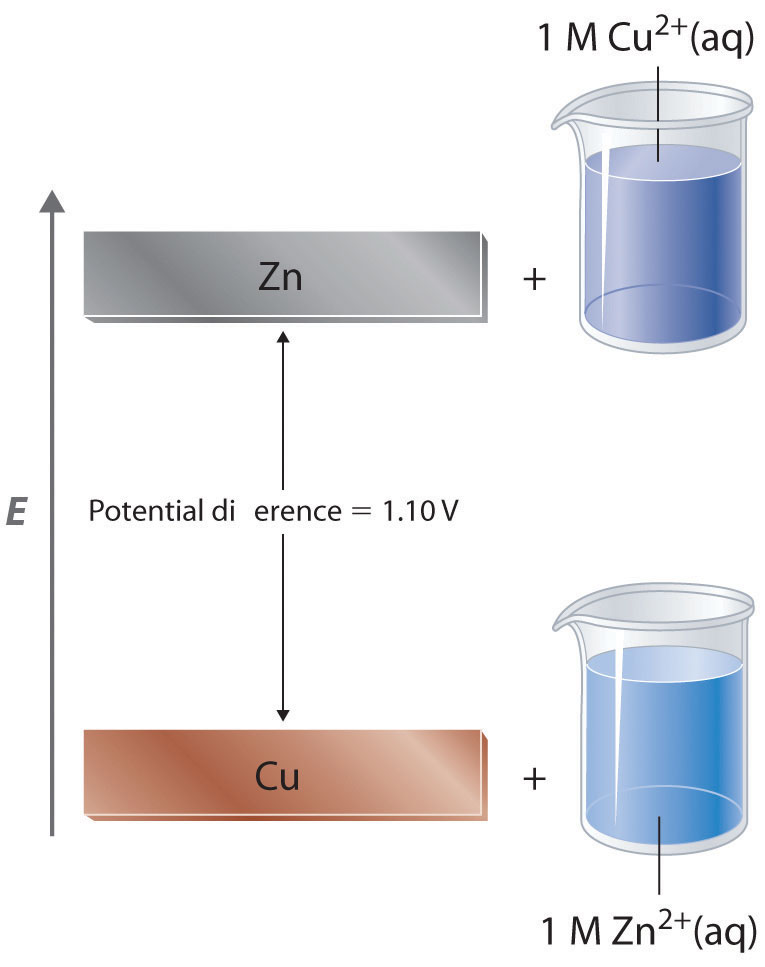
The standard electrode potentials of Zn and Ni respectively are - 0.76 V and - 0.25 V. Then the standard emf of the spontaneous cell by coupling these under standard conditions is:

Chemistry - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different elements